…the impact of covid-19 on rare diseases…
Stephen Tsoroti
In March 2020, Kamwala Township south of Lusaka, Zambias capital city wore an ethereal frown.
The deafening yells and shouts from streets vendors were not heard. The often crowded streets were deserted, a creepy air patched every house. It was as if an apparition had visited and declared death upon it.
It was no longer a rumour.
The virus was here and was killing people. For now, the only remedy was to stay indoors,” described Mainess Chileshe.
Adds; “For me, it was the stark reality that our visit to the University Teaching Hospital (UTH) will be restricted, and even if I go, the chances of getting the regular supply of moisturizers and ointment needed for my child will not be there.”
Since then, the situation has not changed much.
“We are still struggling to get the basic treatment for our child,” says Chileshe.
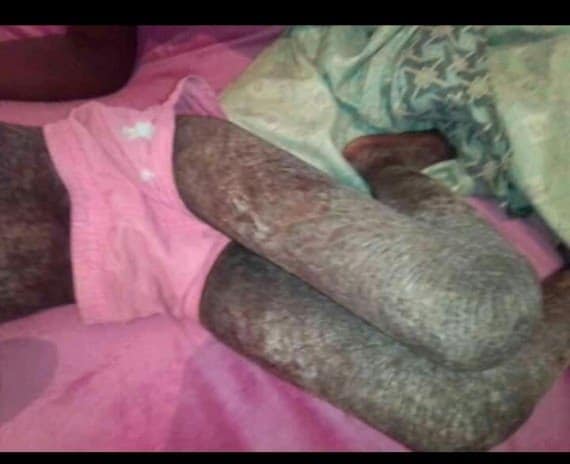
At the dawn of COVID-19 in Zambia in 2020, Chileshes daughter Peggy 6 years old, had been diagnosed with ichthyosis vulgaris an inherited skin disorder in which dead skin cells accumulate in thick, dry scales on the skin surface.
The scales also called fish scales disease or fish skin disease can be present at birth but first appear during early childhood.
Though not contagious. It is a life condition, and a genetic disorder which occurs 1 in 10,000 people.
Doctors at UTH prescribed hydrocortisone ointments and recommended that the family buy creams to keep her skin moisturized at all times.
Meanwhile, for Young Kumbirai Nhondo* in Zimbabwe, COVID-19 was a body blow he could not survive the waiting, as he succumbed before help could arrive.
After years of mis-diagnosis physicians found the cause to be Mucopoysaccharidosis (MPS), is a rare disease in which the body does not have enough of an enzyme needed to breakdown long chains of sugar molecules and remedied a drug.
The problem was that the medicines were not available in the country and the family decided to source the drugs from USA.
With virtually the entire world under lock down Nhondo’s medicines came months late after he had succumbed to the disease.
Since the COVID-19 days, people living with Rare Disease (RD) in southern Africa have been disillusioned.
Their stories-punctuated by sad encounters of not having access to healthcare services, clinical trials being halted, patients visit to health care facilities or trial sites not possible due to risk of COVID-19 infection, health care providers busy caring for covid-19 patients and unable to carry out day to day clinical trials duty, many laboratories for scientific have been closed due to COVID-19 public health concerns.
Trudy Nyakabangwe head of the Rare Diseases Foundation in Zimbabwe portrays situation as dire as the pandemic has stretched families seeking treatment.
She says through her organizations work, families have been struggling to get diagnostic tests, get treatment and access care services as COVID-19 restrictions take center stage.
“It’s heart rending, we have to find fast solutions to arrest the situation.”
Nyakabangwe feels ways and means should be found quickly to simplify the tortuous regulatory process that pharmaceutical wholesalers, NGOs and health institutions must undergo to bring drugs to market to help southern Africa consumers of life saving drugs.
Pediatric and Consultant at Parirenyatwa Hospitals Pediatric unit in Harare Loyce Hlatwayo depicts COVID-19 as a barrier to diagnosis, treatment and drug supply.
However, sees the lack of statistics around RD as an impediment to drug supply issues.
“We have to count all the cases for us to get a total picture of rare conditions in the country and region.”
Hlatwayo contends that whereas the country and region has done much in communicable disease, the availability of specialized diagnostic equipment and registry can produce different results.
“It is ineffective to deal with the bane of RD and the drug supply side without addressing the records and registry challenges,” challenges Hlatwayo.
Heam-oncologist Hlatswayo indicates that rigorous effort needs to be concentrated on finding the numbers, pushing for proper records and putting up a registry to inform stakeholders to ease the drug supplies side as well as showing the trends of rare conditions in the region.
Melissa Haendel university of Colorado Anschutz in the USA is of the same mind, knowing how many RD are, is critical in order to know their treatment regime. If rare diseases are not counted rare disease patients will not count.
To know how many rare diseases there are, clinical systems require a common set of definitions to best aid diagnosis and care.
“We need publicly available information about individuals with rare diseases because better counting of rare diseases will lead to better patient outcomes,” says Haendel.
Generally, life threatening, chronic and incapacitating RD have not been captured well in national records of southern African countries, leading to health experts, researchers and care givers concluding that lack of data is a barrier to the procurement of RD drugs.
On the other hand, drug manufacturing and pharmaceutical companies worldwide have favored volumes and profit in the supply of drugs, but the same companies claim that drug development also requires patient recruitment in clinical trials, regulatory requirements, profitability and sustainability.
Unlike countries in the west where robust generic industry exists, only one country in Africa, South Africa, has made strides in becoming a high tech hub of pharmaceutical distributor and manufacturer of generic drugs.
But South Africas pharm market, and local production scene is dominated by generics mostly focused on the countrys more prominent killers HIV/ AIDS, tuberculosis TB and hepatitis A and B, as well as common medications for influenza, pain and other everyday ailments.
That scenario leaves Zimbabwe and its neighbors under severe pressure to import from elsewhere, where the process becomes expensive and cumbersome for people with RD.
Battered economies and deteriorating health facilities and service infrastructure, also worsens the southern African countries abilities to procure drugs for its people.
Local health experts say the will to combat RD will also hinge on the governments prioritizing non-communicable diseases.
Which means countries like Zimbabwe, with enabling local drug regulatory bodies such as the Medicine Control Authority of Zimbabwe (MCAZ), can do much to advocate and recommend funding for research or procurement of life-saving drugs.
Perhaps coming late though, the MCAZ intends to discuss with various patients interfacing bodies to understand the needs of members of community that need guaranteed access to life-saving medicines. “We are aware of the supply challenges that have come as a result of COVID-19 pandemic,” says MCAZ Projects and Public relations officer Shingia Gwatidzo.
“It makes sense at the moment to advocate for more drugs importation, but in future we need more recognition at home and internationally that patients with rare conditions needs critical attention,” maintains Nyakabangwe, “If we have a goal to attain universal health, then all people need to included.”
According to the International Rare Diseases Research Consortium Assembly, 500 rare disease drugs have reached the market, and there are 700-800 treatments in development. Worldwide, 300 million people worldwide are known to have a rare disease.
Anne Pariser Managing Director office of Rare Disease Research National Centre for advancing translational sciences NH, warns — each year 200 to 250 new rare diseases are being discovered, of which 50 percent of them first manifest in children. How much in Africa? – is a big guess.
Meanwhile, Chileshe pins her hopes on improved supply of medicines, hoping COVID-19 behaves well.
-First published on Zambian Eye
*correct names of source changed to protect their privacy